Executive Summary
The healthcare information technology ecosystem is always evolving. Concepts such as digital health, digital transformation and healthcare interoperability are frequently used to describe trends and the future of what traditionally has been called healthcare IT. GeBBS continuously monitors developments and evaluates their impact on its strategy, products, and customers.
Even within payer organizations, digital transformation can have many facets, from core business operations to administrative functions such as human relations. Arguably the most significant digital transformation under way pertains to core payer operations driven by the changes in clinical data interoperability. One of the latest of those is FHIR® (Fast Healthcare Interoperability Resources: pronounced “fire”), which is a standard for exchanging healthcare information electronically using web technologies.
In this paper we seek to clarify what we mean by true interoperability in healthcare IT, while presenting a comprehensive view of how the need for and access to structured clinical data will soon drive a dramatic shift in payer operations.
In GeBBS’ strategic assessment, several highly significant changes are happening in the healthcare IT ecosystem. Specifically, regulations related to interoperability and other policies, as well as business and market forces, will change how payers will need to operate in the foreseeable future. Interoperability is already changing how patients participate in and take control of their own health and healthcare, and that is just one of many changes yet to come.
The shift in interoperability will fundamentally change how payers operate internally and how they interact with external stakeholders, especially providers. In part that will be to implement:
• Innovative business models, such as value-based care, in which providers strive to improve health outcomes for populations under their management while at the same time reducing costs for those populations.
• New payment models, such as value-based payment, in which providers take on more risk and share in cost savings from their value-based care efforts (known as upside risk or pay for performance) or providers share risk both ways—in savings and potential losses (known as downside risk).
In large part, adoption of these collaborative business and payment model initiatives will be driven by the necessity for organizations to stay competitive. Providers and payers both will need to execute them while remaining compliant with regulatory mandates, and achieving our Quintuple Aim:

Assessing the Key Drivers of Change in Digital Transformation
With a significant footprint in the provider space and core capabilities and assets that also apply very well in the payer space, GeBBS is in a unique position with its deep understanding of key stakeholders. In this paper we start with our assessment of key drivers of our digital future. Drivers for change can be categorized in two ways:
• Enabling factors, which are typically regulations and technology that make change possible.
• Compelling factors, which are compliance and business imperatives that make it necessary or at least good business sense to implement changes.
Business Models
To date, the most significant driver for more timely clinical data has been the shift in business models including value-based and risk-sharing arrangements. Those represent steps in the right direction, but typically are implemented as one-on-one solutions, often with non-standard data content and transport protocols, thus adding to the complexity and costs of clinical data operations. While the business case justifies such investments, they can also be satisfied with the emerging, standards-based structured data, thus presenting an area of improvement and simplification.
Enabling Technologies
The technologies that are driving the digital transformation in this case did not have to be invented. This is in part because they already existed in the broader IT ecosystem. This includes:
• Internet connectivity;
• Application programming interfaces (APIs) that help computer systems exchange information securely over the internet, such as RESTful APIs;
• Open-authorization security standards, such as OAUTH2, which allow websites and apps to share access to resources;
• and much more.
This digital transformation is due to the wise and pragmatic adaptation of the healthcare domain-specific implementations of those technologies in the key standards being rolled out and mandated—most significantly Health Level 7 (HL7) International FHIR data and API standard—in the applicable regulations.
Regulations
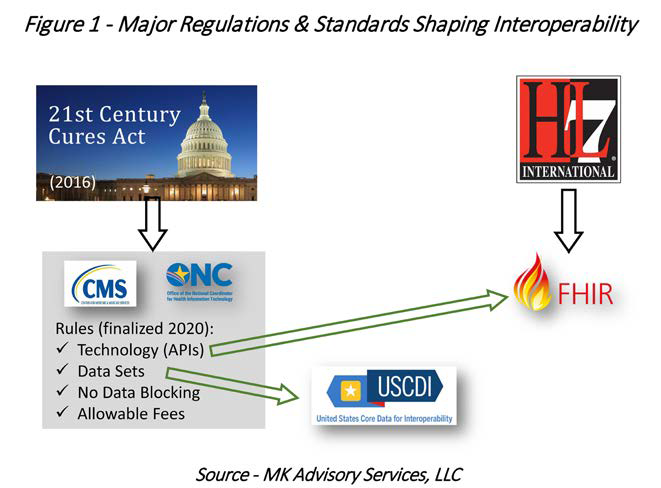
Most relevant new regulations stem from the 21st Century Cures Act, passed in 2016. One key theme in the law is to allow patients to access their own health data “without special effort” and free of charge. The detailed rules have significance well beyond the patient, because what is mandated for the patient, first and foremost, also becomes valuable to other stakeholders, including payers. Specifically, rules from the Office of the National Coordinator for Health information Technology (ONC) and the Centers for Medicare and Medicaid Services (CMS) resulting from the Cures Act have been finalized and in full effect as of Q1 2023.
These include:
• API and data standards and technology,
• Data content and quality minimum requirements, and
• Creating a regulatory framework to compel data sharing and no or low fees.
The regulations in this context are both enabling and compelling factors. Regulations that apply to provider systems and technology developers (primarily the ONC interoperability rules) are very much enablers. The fact that every provider system will expose a standards-based API for clinical data, that it cannot block access to that data, and that the fees it can charge are “reasonable” and uniform are clearly huge leaps forward. Payer organizations are not required to use these APIs, but doing so will have significant benefits. Best practices, planning and ROI modeling tools are being developed to add hard numbers to a logical argument for adopting those APIs as sources for clinical data. This is further presented in the Impact section. Some regulations mandate standards, which are explained next and in Figure 1.
Standards
The standards relevant to interoperability (and related use cases) are governed primarily by HL7 International—FHIR, FHIR Implementation Guides, and FHIR-Clinical Quality Language (FHIR-CQL). It is not necessary to discuss the details of these standards to make the key point that having a standard mandated is key.
While our industry will continue to have discussions about which standard is “best” for which purpose, it is certainly best for the entire ecosystem to standardize on one “language” to make communication easy, frictionless, and ultimately almost effortless.
Of course, FHIR is not the answer to all our clinical data needs, at least not by itself. While FHIR provides the transport mechanism (API calls) and the content formatting, it does not mandate what data elements need to be provided to the recipient of the data. That is where U.S. Core Data for Interoperability (USCDI) comes in. Managed by ONC, it specifies which data elements are mandatory when responding to a FHIR API call.
This is significant because different use cases have specific minimum data requirements. While the earliest versions of USCDI are not comprehensive vis-à-vis certain use cases, the versions in development (they are versioned, rolled out and mandated annually) are very much aiming to ensure use-case “coverage.”
The collaboration between ONC and the use case advocates/stewards—e.g., National Committee for Quality Assurance (NCQA) for Healthcare Effectiveness Data and Information Set (HEDIS) quality measures, and HL7, with its implementation workgroups for use cases such as prior approvals and gaps in care—is essential and is happening to accomplish that important goal.
Competitive & Cost Reduction Pressures
Even though the above drivers are compelling and will require the adoption of the digital operations paradigm, the overarching driver will be the need to stay competitive. This is accomplished by optimizing revenue, reducing costs in operations and care, and also by performing functions such as member services, quality management and reporting at a level that is competitive as measured by customer satisfaction ratings, CMS Star Ratings, NCQA quality scores and other key performance indicators.
Payer organizations always face cost reduction pressures. Making this digital transformation succeed can significantly reduce costs for building and maintaining IT systems to handle disparate clinical data. Complete, structured and standards-based real-time data will also impact costs of care. Timely member outreach, assistance and intervention have been shown to significantly reduce occurrences of complications and the corresponding costs (e.g., of hospital readmissions).
Fundamental Ecosystem Shift
The above factors, along with innovative solutions, will likely lead the clinical data ecosystem to a tipping point, where old business models and possibly entire categories of products and services will not be viable anymore. (Note: clinical data ecosystem is a term defined by MK Advisory Services as the actors and clinical data exchanges between those actors in the U.S. healthcare system.) With the volume of clinical data ever increasing, unit costs significantly reduced, and real-time access eventually being table stakes, one can imagine how this ecosystem shift will play out. It can be expected that the primary stakeholders (patients, providers and payers) will significantly benefit from such a shift, this does have implications for strategy and planning and should be considered.
Payer Functions and Operations Transformation
With enabling technologies and regulations in place, regulatory mandates forcing changes in payer models, and significant business opportunities compelling a more strategic digital transformation of payer operations, the question becomes: which functions and departments are impacted and what does that look like?
There are a number of use cases that should be considered and may vary from organization to organization. The following typically exist at a payer organization and should be considered:
• Quality reporting
• Quality operations
• Risk adjustment
• Care management
• Care coordination
• Population health
• Gaps in care
• Patient outreach
• Provider communications/relations
• Fraud, waste and abuse
Example: Digital Quality
The transition to digital quality is the most concrete example. Not only have CMS and NCQA, the most important stewards of quality programs, defined a clear path toward digital quality with digital quality measures (dQMs), they have already declared FHIR-CQL as the new standard for all measures going forward, their intent being to discontinue so-called paper measures (measures where the measure rules are documented by them but implemented by various vendors with technologies of their choosing). In addition, they have designated the FHIR standard as the paradigm for all data to be used in measuring quality.
This has significant implications for health plans:
• Less reliance on administrative data. As measures evolve to be more “meaningful,” administrative or claims data, typically easy to access as it resides within the payer organization, will be de-emphasized with the implication that future measures will rely more heavily on clinical data (typically to be retrieved from outside the payer organization).
• Phase-out of hybrid measures. These measures today use a combination of administrative and clinical data. The clinical data to date is being collected through a Medical Record Review (MRR) process, which is highly manually entered, fraught with uncertainty and problems, and very costly on a per-member basis. To make this process manageable at all, hybrid measures only measure a random sample (411 members), regardless of the size. With the shift to digital quality measures, this method is being phased out and replaced with measures that will require clinical data for an entire population.
• More clinical data. The changes above will drive a significant increase in required clinical data. Not only will that data need to be sourced but also must be sourced at a similar or lower cost. In addition, with data being available in real-time, it will be ever more important to leverage that data much more effectively to close care gaps and perform other quality management functions to remain competitive with peers in quality ratings and programs like CMS Star Ratings.
• Changing data validation and audit processes. Structured, standards-based data enables much more streamlined and automated validation, solving a big pain point in the quality measurement ecosystem. There are a number of layers to this, from validating structure to use of proper code sets such as ICD-10 Diagnosis Codes and Logical Observation Identifiers Names and Codes (LOINC), to source validation (data provenance).
Example: Digital Risk Adjustment
Barely talked about in this context to date is risk adjustment. Even so, with the more common practices of concurrent and prospective risk adjustment reviews, plans and vendors are adopting practices to source data and medical charts more frequently and are using optical character recognition (OCR) and natural language processing (NLP) technologies to streamline the structuring and analysis of that data.
Gleaning the approach and lessons that can be learned from the upcoming NCQA Digital Quality Transition initiative, the parallels between risk adjustment and HEDIS become apparent. The MRR process used in risk adjustment is almost identical in workflow to the that for HEDIS hybrid measures, and equally laborious and costly, even though automation with OCR and NLP has had an impact.
However, just as the availability of standards-based, structured clinical data makes digital quality possible, so will it enable a transformation in the risk adjustment realm, including addressing the following issues:
• No More Suspecting. With the availability of real-time clinical data and significantly lower unit costs for clinical data, it is possible that sourcing all clinical data for a membership on an ongoing basis is more cost-effective than the traditional suspecting process (This is to be verified with ROI modeling—see Framework on page 10). When sharing clinical data across multiple use cases the ROI becomes significantly more compelling for each (see Clinical Data Operations in the next section for more).
• Automated Audits. Perhaps considered quite the stretch today, the possibility of having automated audits may not be too far in the future. Once data provenance has been automated (a number of components need to be put in place, but ONC and other organizations are already working toward that), FHIR endpoints and USCDI will make it possible to automatically validate the source and validity of data. This has of course significant implications for audits in terms of volume, speed, detail, and accuracy. Once automated, technology will enable auditing risk adjustment submissions in real time. How much and exactly how audits will work is going to be driven by policy, not by technical limitations, logistical constraints, or prohibitive costs.
• Changing Role of NLP. Similar to coders in MRR, many steps performed in a typical NLP process (including OCR) will become obsolete. Depending on the use of standard terminology in notes fields, NLP can still have a very important, more specialized, and narrow application, possibly in combination with the specialized reviewing process described in the previous point. Overall, the process will be more streamlined with effectively eliminating the part of the NLP process that handles most variability—the many possible formats chart images can take and the OCR/NLP process needs to recognize and handle.
• De-facto Elimination of MRR. With all clinical data being structured and standards-based, a big portion of the medical records review becomes obsolete. (i.e., identifying the relevant parts of the chart and entering them into a form and manually transforming unstructured into structured data). This not only saves significant costs for manual labor, it also speeds up the process to essentially instant processing and further eliminates the possibility of human error. While some review of notes fields or edge cases may still be required pending any additional innovations on standardizing coding logic (Da Vinci Initiative, 2023)—it will become a rare exception review process performed by a highly experienced coding expert.
Other Payer Functions
The examples above provide a good indication of how to think about other use cases and how standards-based, structured clinical data can and will transform them. Especially, the potential and eventual realization of obtaining all relevant data in real-time will have significant impact on other functions such as care coordination and member outreach.
What emerges as the centerpiece to all of this transformation is how clinical data is sourced and handled.
Clinical Data Operations
The need and benefits of managing clinical data in a centralized and standardized way become clear when it is possible to obtain good, structured data fast enough to satisfy the most time-critical use case and then use and re-use it for any and all use cases. This eliminates potentially massive duplication and complexity within the organization. It will also allow the central and increasingly automated and coordinated management of all external data source, both during the transition to connecting with external FHIR endpoints and certainly once all sources are FHIR-based.
The most common term and concept for this is clinical data integration (CDI). This effectively combines the concepts of centralizing and standardizing clinical data for a payer organization for further use by a variety of “subscribing” use cases such as quality management, quality reporting, risk adjustment, and more.
The benefits of implementing CDI are many for any payer organization, even without the evolution of interoperability. However, in the context of the digital transformation, strategically approaching clinical data is even more important and beneficial not only to streamline and optimize internal data operations, but also to manage the upcoming transition of external data sources. In the short term, that means taking advantage of FHIR endpoint opportunities in a point-to-point connection approach. In the longer term, it also prepares the organization to dynamically source clinical data from any source.
It is important to plan a CDI initiative with such an end state in mind to truly take the optimal approach and transform a payer organization to perform optimally in the foreseeable future.
Impact of the Interoperability Transformation
The impact of the interoperability shift will affect everyone participating in the clinical data ecosystem. Although this paper’s focus is the payer organization, it does mention patients and provider organizations as affected stakeholders. The focus of this section will remain on the payer organizations.
Payer Organizations
Before long, payer organizations will have no choice but to adopt at least some of the digital changes to remain compliant with programs such as digital quality measures. Beyond that, it can be anticipated that plans who do not embrace the digital paradigm will face significant operational disadvantages in relation to their competitors.
On the flip side, the opportunities created by the new digital paradigm go well beyond compliance and staying competitive. Payers have the opportunity to transform their operations with significant efficiencies, better clinical outcomes and cost savings, and optimized revenue.
As shown in Figure 2, there are a number of functions/use cases that will each individually benefit from implementing interoperability with standardized, structured clinical data. Most significantly, however, the organization as a whole will realize transformational benefits and tangible ROI when a comprehensive clinical data strategy is implemented for the entire organization.
Payer organizations will see improvements (ROI) in three major areas:
• Operational. Standardization and automate will streamline processes both for data acquisition and processing as well as related business processes and workflows, significantly reducing costs in both setting up and maintaining data relationship.
• Clinical. Standardized data available increasingly in real-time will transform how care is coordinated, how quality is measured, and how gaps in care are identified and closed, therefore keeping members healthier.
• Financial. While operational streamlining drives down costs in setting up and maintaining systems and departmental activities, the clinical benefits also will significantly drive down the cost of care on multiple fronts. A third source of cost savings will be the reduced costs of acquired data due to automation and regulations. Finally, better, more timely and complete data will allow plans to optimize revenue in multiple ways, including incentive payments and risk adjustment.
Vendor Ecosystem
With the standardization and “liberation” of clinical data, interoperability will shift how data is discovered and accessed. That means that entire vendor categories that have performed needed functions will become obsolete and/or need to pivot their offerings (Gartner, 2021).
For example, companies that provide proprietary EMR access and map data for payers will have a significantly reduced importance in a paradigm where access is standardized and mandated, and one standard data format will make mapping unnecessary.
Similarly, aggregators such as health information exchanges (HIEs) will be less important in providing data from many different sources once these sources are easily identified and accessed directly, likely at a lower cost, with less delay, and better security, auditability, and data validation capabilities.
Data Discovery & Access
In a future ecosystem where all clinical data is accessible via standard APIs, many of today’s solutions and tasks will become unnecessary, as explained above. But as big a leap forward as this standardization is, it does not provide all the pieces needed to truly dynamically discover and access clinical data wherever it resides. That includes a patient-level record locator (as opposed to FHIR endpoint directories that just list the “address” of FHIR APIs, which is also an important foundational capability) and the ability to access data sources without first having to establish a contractual relationship between parties (i.e., dynamically obtain permission to access a FHIR API).
However, these capabilities are already being developed and are ready to be deployed when the necessary components of the clinical data ecosystem have matured sufficiently. Velox Health Metadata Inc., a GeBBS partner organization, has developed these capabilities and an operating model to provide patient level record locator services and other capabilities to enable the dynamic Clinical Data Ecosystem.
Managing the Transition to the Digital Future of Payer Operations
As payer organizations recognize this upcoming shift toward Digital Payer Operations, the question becomes: “How does one approach this transition?” Just as with any change effort, especially a strategic one, the approach is to:
1. define the scope,
2. define the goal or end-state,
3. assess the current state,
4. create a plan to get from here to there, and
5. implement the plan.
Implementation becomes much easier with a framework, best practices and benchmarks to rely on. Our industry has been developing some of those (e.g., FHIR Implementation Guides for various use cases by HL7 workgroups like DaVinci) and more are in development. In addition, the following framework is very useful to frame this digital transition.
Framework
Velox Health Metadata Inc. a GeBBS partner organization, has developed this framework and a comprehensive implementation assessment, management and tracking platform. A framework to enable payer organizations to successfully make this transition handles a number of key aspects. The following description summarizes the framework, which:
• inventories all existing data flows and use cases;
• identifies available FHIR data sources on an ongoing basis;
• facilitates (and eventually automates) switching to FHIR data sources;
• captures current costs and potential savings;
• captures investment estimates;
• projects ROI and prioritizes individual initiatives to maximize impact;
• continuously tracks investments and progress; and
• measures realized ROI.
The framework is based on breaking down the clinical data problem domain into two areas:
1. Internal—Clinical Data Integration (CDI). This includes all clinical data and systems within the payer organization that are considered within the scope of the transition. Payer organizations control all these assets and can deliberately act on streamlining and consolidating how clinical data is handled and processed inside the organization. The primary task toward “going digital” internally is to implement a centralized way of handling all clinical data and sharing it appropriately with all the functions/systems/use cases.
2. External—Clinical Data Ecosystem (CDE). Includes all sources, aggregators and processors of clinical data that are outside the payer organization. While the payer does not control these resources, being able to have an inventory of relevant resources and the ability to leverage them toward the transition is key.
By separating what a payer can and cannot control and at the same time seizing the external opportunities right when they emerge, payer organizations can constantly execute and adapt while continuously working toward the goal of sourcing all standards-based structured clinical data (FHIR endpoints) in real time, continually realizing ROI and gradually transforming their operations.
Timeline
While there are a number of factors that are not entirely predictable, there is overwhelming momentum toward the clinical data ecosystem described above. The transition is well under way. This may seem optimistic from a payer’s perspective, simply because the compliance efforts that had to be completed to date do not make obvious the potential they represent. But all the interoperability mandates from CMS and ONC are in effect as of Q1 2023.
Assuming that the FHIR adoption will continue to track as outlined above from the HL7 Survey and the projection for 2025, payers can assume that the transition can be completed as early as 2026 and we expect that “early adopter” payer organizations will very much attempt and accomplish that.
Conclusion
The digital transformation for payer organizations is upon us. While initiatives to date may give the (false) impression that adopting standards-based structured clinical data is about necessary costs just to stay compliant and avoid fines, there are massive benefits and opportunities that hide behind these compliance efforts.
An entire clinical data ecosystem is being transformed under our noses, but it is not obvious until we start taking advantage of that new ecosystem. FHIR endpoints are about to be ubiquitous, and they will enable accessing clinical data everywhere in one format in real time. That is truly transformational and arguably a once-in-a-generation opportunity.
This is with the understanding that this transition will be a multi-year effort, that additional mandates will likely take effect in the next two to three years and, most importantly, that there is provable ROI implementing a CDE strategy. With each switch to a FHIR-based data source as well as a significant competitive advantage or at least parity with market leaders, all payer organizations should begin the assessment and planning stages for this digital transformation right away.
Use cases, including digital quality, are rapidly evolving, creating the need for FHIR-based data and the necessity to source more complete clinical data in a timely fashion while minimally maintaining and possibly reducing costs of data acquisition.
Above all, payer organizations should be very clear about the fact that this transition is not primarily about compliance, but about opportunity. Opportunity to improve operational functions, improve and simplify data operations, and opportunity to reduce costs, including costs of care, which of course means the opportunity to improve the health and lives of members and the healthcare system overall. Ultimately, this is a win-win-win for all stakeholders, for advancing our healthcare systems on our Quintuple Aim—better health outcomes, improved patient experience, reduction in costs, clinician well-being, and health equity.






